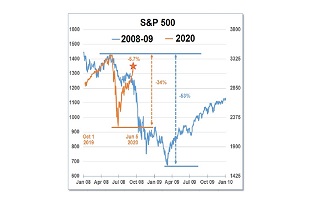
eResearch | This week, the Trump administration ordered more than 500,000 doses of Remdesivir, the first drug in the world approved for treating COVID-19, developed by Gilead Sciences, Inc. (NASDAQ: GILD). This event cleared out Gilead’s supply for July and 90% of Gilead’s expected productions for August and September, leaving no supply for the rest of the world.
 Dr. Andrew Hill, a senior visiting research fellow at Liverpool University, stated, “there is a way for the U.K. to secure supplies of this and other drugs during the pandemic, through what is known as a compulsory license, which overrides the intellectual property rights of the company. That would allow the U.K. government to buy from generic companies in Bangladesh or India, where Gilead’s patent is not recognized.”
Dr. Andrew Hill, a senior visiting research fellow at Liverpool University, stated, “there is a way for the U.K. to secure supplies of this and other drugs during the pandemic, through what is known as a compulsory license, which overrides the intellectual property rights of the company. That would allow the U.K. government to buy from generic companies in Bangladesh or India, where Gilead’s patent is not recognized.”
This event brings strong implications for pharmaceutical companies who rushed to develop vaccines, but may not have the necessary capabilities to produce enough supply for the world. Nevertheless, Remdesivir is only a treatment to facilitate recovery and not a vaccine to cure COVID-19; it is yet to be approved by the U.S. Food and Drug Administration (“FDA”) as it only received Emergency Use Authorization (“EUA”) for the drug.
Once a vaccine is developed, there are several concerns. Will Trump secure all of the supply? Will supply and production capacity be sufficient to reach demand? Will governments be forced to implement compulsory licenses?
Remdesivir
Remdesivir was originally developed for hepatitis C, repurposed and studied for treatments in Ebola, and now approved to treat COVID-19 through EUAs in the U.S., India, Singapore, Japan, and the U.K.
Remdesivir is priced at US$390 per vial for governments of developed countries, which equates to US$2,340 in total cost per patient, as six vials are needed per treatment program. In the U.S., the same government price of US$390 per vial will apply, but insured patients are expected to pay US$520 per vial, a 33% premium due to intermediary costs.
Earlier this month, Gilead announced results from its Phase 3 trial that tested Remdesivir on patients with moderate COVID-19 pneumonia, which found treatment groups 65% more likely to have clinical improvements. However, the results have yet to reach statistical significance.
COVID-19 Vaccines in the U.S., Canada, and China
In the U.S., Pfizer Inc. (NYSE: PFE) and BioNTech SE (NASDAQ: BNTX) partnered to jointly develop a vaccine for COVID-19 called BNT162. This month they received US$113 million in financing to support development and scale up manufacturing. BioNTech, the first European company to release vaccine trial results, will provide Pfizer with the clinical supply of BNT162 from GMP-certified mRNA manufacturing facilities in Europe.
 Last month, Pfizer ran a phase 1/2 clinical trial for the vaccine with 24 people in the U.S., which showed “significant elevated” antibodies within four weeks of first injections. Based on these results, Pfizer and BioNTech expects to launch a larger U.S. trial with 30,000 patients within the next couple of weeks, subject to regulatory approval.
Last month, Pfizer ran a phase 1/2 clinical trial for the vaccine with 24 people in the U.S., which showed “significant elevated” antibodies within four weeks of first injections. Based on these results, Pfizer and BioNTech expects to launch a larger U.S. trial with 30,000 patients within the next couple of weeks, subject to regulatory approval.
In Canada, Medicago Inc. (TSXV: MDG) is expected to become the first company with a Canadian-made vaccine candidate to enter human trials, supported by research teams at Laval University, and funds from the federal government and Quebec.
Last month, Medicago’s early animal testing of the COVID-19 vaccine yielded positive antibody responses within 10 days of dose injections. Once regulatory approvals are received, Medicago expects to run Phase 1 trials in the next couple of weeks, with hopes of Phase 2 trials launching in October.
 In China, CanSino Biologics Inc. (HKG:6185) received approval for use of its COVID-19 vaccine candidate called Ad5-nCoV, which showed capabilities to prevent diseases caused by COVID-19 in Phase 1 and Phase 2 trials.
In China, CanSino Biologics Inc. (HKG:6185) received approval for use of its COVID-19 vaccine candidate called Ad5-nCoV, which showed capabilities to prevent diseases caused by COVID-19 in Phase 1 and Phase 2 trials.
CanSino Biologics developed Ad5-nCoV through a partnership with the Beijing Institute of Biotechnology, a research unit part of the Academy of Military Medical Sciences. Last month, China’s Central Military Commission approved the use of Ad5-nCoV on military personal for a period of one year.
U.S. Food and Drug Administration
 This week, the FDA announced a guidance with recommendations called the “Development and Licensure of Vaccines to Prevent COVID-19”, to facilitate timely, safe, and effective development of drugs related to COVID-19.
This week, the FDA announced a guidance with recommendations called the “Development and Licensure of Vaccines to Prevent COVID-19”, to facilitate timely, safe, and effective development of drugs related to COVID-19.
To facilitate faster development of a COVID-19 vaccine, the FDA has provided EUAs for 157 tests, including 132 molecular tests, 24 antibody tests, and 1 antigen test.
The pharmaceutical industry in not competing against each other to reach a COVID-19 vaccine first, but rather, companies and governments are collaborating to get as many vaccine candidates as possible into trials, to ultimately create the highest chance of discovery for a legitimate COVID-19 vaccine.
eResearch recently published several articles regarding public companies focused on COVID-19, including:
- April 22, 2020 – “Large Cap Public Companies Developing Drugs and Diagnostics Tools for COVID-19”
- April 27, 2020 – “Public Canadian Companies Developing Tests and Vaccines for COVID-19”
- May 2, 2020 – “Canadian Public Companies Focused on Prevention and Protective Equipment for COVID-19
//