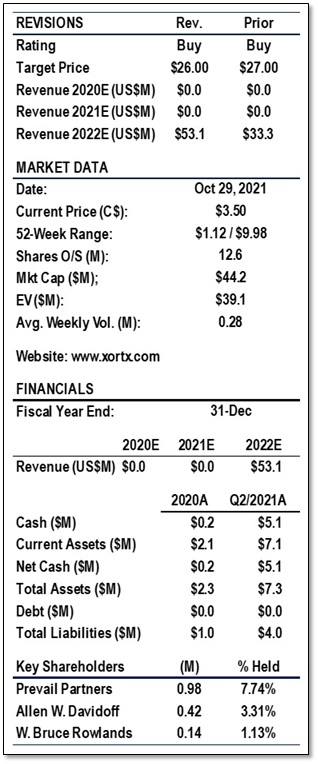
Based on our revised numbers, the total enterprise value doubled but the large share increase has kept a target price to C$26.00, a slight decrease from our previous target price of C$27.00 (share consolidation adjusted). We reiterate our BUY rating.
COMPANY DESCRIPTION:
 XORTX Therapeutics Inc. (“XORTX” or “the Company”) has developed a proprietary drug delivery platform through innovative formulation technology that is designed to optimise Oxypurinol formulations to substantially improve the drug’s features and increase the bioavailability of the drug. These new formulations and new chemical entities are underdevelopment to increase the oral dosing range and may reduce the side effects of the original drug as well as support increased patient compliance.
XORTX Therapeutics Inc. (“XORTX” or “the Company”) has developed a proprietary drug delivery platform through innovative formulation technology that is designed to optimise Oxypurinol formulations to substantially improve the drug’s features and increase the bioavailability of the drug. These new formulations and new chemical entities are underdevelopment to increase the oral dosing range and may reduce the side effects of the original drug as well as support increased patient compliance.
INVESTMENT HIGHLIGHTS:
 Very simply:
Very simply:
- XORTX is a research and development company optimising delivery of existing drugs to treat specific patient populations with kidney disease. Frequently this increased bioavailability is accompanied by increased tolerability, efficacy, and promotes compliance.
- XORTX is using its optimisation technology to develop multiple drug treatments without incurring the high cost of research.
- XORTX is taking advantage of Oxypurinol to develop two unique proprietary therapies that could help to solve unmet medical needs related to aberrant purine metabolism and elevated uric acid, an important health biomarker involved in multiple organ diseases such as kidney, liver, heart, etc.
- XORTX’s most advanced therapeutic – XRx-008 – is aiming to be the next drug to be approved for the indication autosomal dominant polycystic kidney disease (ADPKD), an orphan indication with more than 150,000 patients in the USA.
- XORTX has 2 therapies – XRx-008 and XRx-101 close to pivotal Phase 3, registration, clinical trials.
- XORTX is fully funded to rapidly advance toward the approval of its first product within the next 18-24 months.
- Balance Sheet Strengthened
- The recent financings, which netted around C$21 million, have largely de-risked the Company and increased the value of its assets.
- It provided the resources for the documentation completion and initiation of Phase 3 clinical trial of both drugs: XRx-101 for the treatment of acute kidney injury (AKI) found in COVID-19 patients and XRx-008 for autosomal dominant polycystic kidney disease (ADPKD).
- XRx-101 could be approved within the next 18 months while XRx-008 could have an orphan drug indication on the market in 2025.
FINANCIAL ANALYSIS & VALUATION:
- Revised One Year Target Price
- Given the de-risked outlook with two major financings, we have reviewed our assumptions to better reflect the future potential target price.
- Additionally, the Company has completed a share consolidation (11.74 for 1 common share) with its US listing. The number of shares has basically doubled in the last year.
- However, moving forward one year and getting close to the initiation of the Phase 3 clinical trials, we feel more secure in reducing our discount rate from 20% to 15% in our DCF model.
- We continue to value the stock using a sum-of-the-parts methodology that includes DCF calculations for the two assets.
- We assign NO value to XRx-225 and believe it will carry significant value when the drug initiates human clinical trials.
- When compared to peer companies, either developing orphan drugs at a similar stage of development or new companies that became public in the last two years, the Company is SIGNIFICANTLY UNDERVALUED.
- Based on our revised numbers, the total enterprise value doubled but the large share increase has kept a target price to C$26.00, a slight decrease from C$27.00 *** previously.
- We reiterate our BUY rating.
You can download the full 25-page report by clicking here: eR-XRX-UR-2021_11_01_FINAL
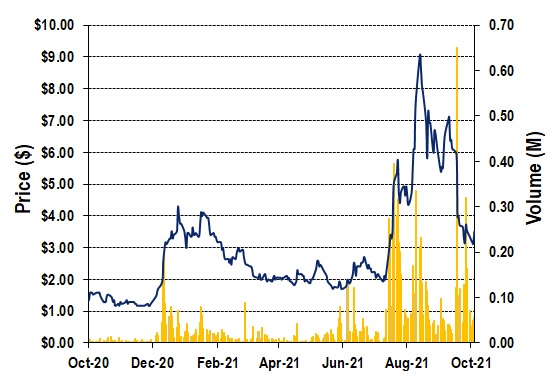
CHART 1: XORTX 1-Year Stock Chart
Notes: All numbers in CAD unless otherwise stated. The author of this report, and employees, consultants, and families of eResearch personnel may own stock positions in companies mentioned in this article and may have been paid by a company mentioned in the article or research report. eResearch offers no representations or warranties that any of the information contained in this article is accurate or complete. Articles on eresearch.com are provided for general informational purposes only and do not constitute financial, investment, tax, legal, or accounting advice nor does it constitute an offer or solicitation to buy or sell any securities referred to. Individual circumstances and current events are critical to sound investment planning; anyone wishing to act on this information should consult with a financial advisor. The article may contain “forward-looking statements” within the meaning of applicable securities legislation. Forward-looking statements are based on the opinions and assumptions of the Company’s management as of the date made. They are inherently susceptible to uncertainty and other factors that could cause actual events/results to differ materially from these forward-looking statements. Additional risks and uncertainties, including those that the Company does not know about now or that it currently deems immaterial, may also adversely affect the Company’s business or any investment therein. Any projections given are principally intended for use as objectives and are not intended, and should not be taken, as assurances that the projected results will be obtained by the Company. The assumptions used may not prove to be accurate and a potential decline in the Company’s financial condition or results of operations may negatively impact the value of its securities. Please read eResearch’s full disclaimer.




