eResearch | XORTX Therapeutics (CSE: XRX; OTC: XRTXF) is a biopharmaceutical company focused on developing drugs that could reduce serum acid uric levels to treat a number of kidney diseases which remains an unmet medical need.
 XORTX does not have any approved drugs but has two potential late-stage drugs in development, each with a proprietary combination using Oxypurinol as the starting ingredient.
XORTX does not have any approved drugs but has two potential late-stage drugs in development, each with a proprietary combination using Oxypurinol as the starting ingredient.
An Initiation Equity Research Report on XORTX was published by eResearch on August 16, 2020: XORTX Breaking Up the Code with Uric Acid
XORTX Clinical Update
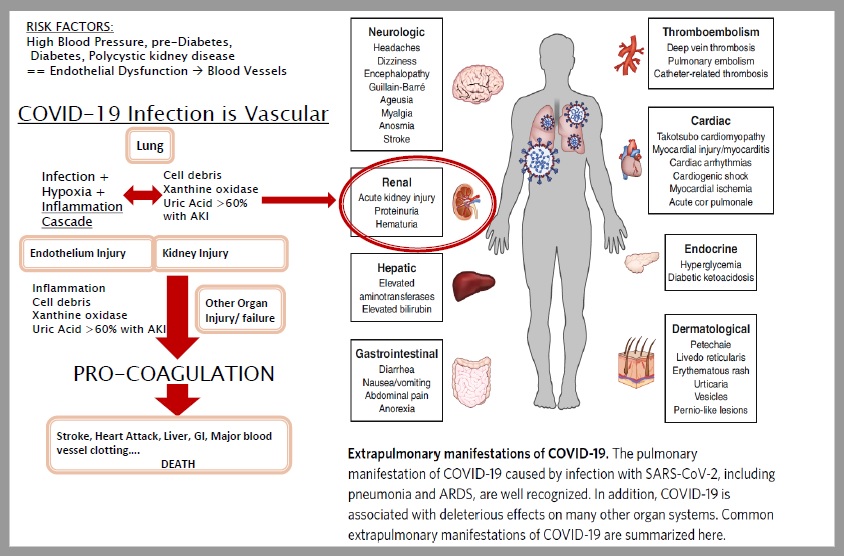
One of the drugs XORTX is currently focused on developing is XRx-101, a treatment with the potential to lower uric acid levels in COVID-19 patients. Lowering uric acid levels in patients could potentially improve Acute Kidney Injury (“AKI”) issues, recovery, and survival outcomes.
Last week, XORTX announced topline results for a clinical study on incidences of AKI with high serum uric acid levels in COVID-19 patients. The study included 7,000 patients, of which 36% experienced AKI during the early onset of COVID-19 with an additional 23% developing AKI during hospitalization. More than half of the patients with AKI had evidence of high serum uric acid levels.
XORTX received positive feedback in October from the U.S. Food and Drug Administration (“FDA”) for the development of XRx-101. XORTX previously filed its pre-Investigational new drug meeting request with the FDA in August to test XRx-101 on COVID-19 patients.
In September 2020, XORTX highlighted a new study related to COVID-19 patients with AKI, which collected data from 3,993 COVID-19 patients in the U.S. The study found that AKI occurred in 46% of COVID-19 patients, with an in-patient mortality rate of 50% amongst those with AKI.
FIGURE 1: Coronavirus – COVID-19 – Progression & High Uric Acid
XORTX Corporate Update
At the beginning of this month, XORTX was invited to the Meridian Clinical Trials Conference. XORTX provided a presentation on its Autosomal Dominant Polycystic Kidney Disease (“ADPKD”) program and its drug, XRx-008.
XORTX was also invited to the World Anti-Viral Congress – COVID-19 and Emerging Diseases Session, to provide a presentation entitled “XRx-101 As a Treatment for Coronavirus – A Strategy to Decrease Acute Kidney Injury, Co-morbidity and Mortality”.
Upcoming catalysts for XORTX include the initiation of a key registration study in Polycystic Kidney Disease and the initiation of a Phase 2/3 study for kidney injury caused by Coronavirus/COVID-19, both expected within the 12 months.
Claude Camiré Senior Equity Research Analyst at eResearch, remarked recently, “With a market cap of less than $10 million and multiple compelling value-creation opportunities in its pipeline, we expect this small cap biopharmaceutical company to become an attractive investment for investors and for commercial partners.
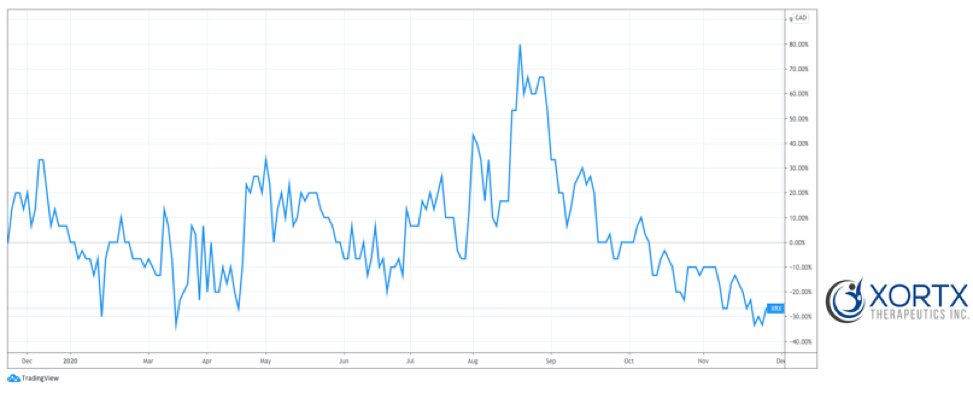
FIGURE 1: XORTX Stock Performance – 1 Year Chart