eResearch| Eupraxia Pharmaceuticals (TSX: EPRX) raised $41 million through the sale of 5.1 million shares. The funds will support Phase II clinical trials of a long-term pain relief treatment for knee osteoarthritis.
 Eupraxia is part of a parade of Canadian biotechnology companies listing in the past year, including Tetra Bio-Pharma (TSX: TBP | OTCQB: TBPMF), Fusion Pharmaceuticals (NASDAQ: FUSN), AbCellera (NASDAQ: ABCL), and Repare Therapeutics (NASDAQ: RPTX)
Eupraxia is part of a parade of Canadian biotechnology companies listing in the past year, including Tetra Bio-Pharma (TSX: TBP | OTCQB: TBPMF), Fusion Pharmaceuticals (NASDAQ: FUSN), AbCellera (NASDAQ: ABCL), and Repare Therapeutics (NASDAQ: RPTX)
Eupraxia had a soft opening and was trading at C$6.00 as of March 22, 2021, below the IPO price of C$8.00
Like many early-stage biotechnology companies, a successful investment in Eupraxia hinges on its lead candidate’s efficacy.
The biotechnology equities market is hot. The iShares Nasdaq Biotechnology ETF (NASDAQ: IBB), ARK Genomic Revolution ETF (BATS: ARKG), and Virtus LifeSci Biotech Clinical Trials ETF (NYSEARCA: BBC) are up 52%, 228%, and 109% in the past year, respectively.
Osteoarthritis Treatment Market
According to the prospectus, knee osteoarthritis treatments are a $5.6 billion market that is currently served by products which have marginal efficacy.
Osteoarthritis is a disease of old age, affecting 10-15% of adults over 60. With an ageing population in North America, more painful knees will likely mean more treatment.
Eupraxia
Headquartered in Victoria, British Columbia and founded in 2012, Eupraxia has several pharmaceutical products in its development pipeline. One of many players developing drug delivery space, Eupraxia is aiming to get the drug to the right place at the steady dose level.
The secret sauce of Eupraxia is its injectable polymer loaded with bioactive molecules. A physician injects the degradable polymer into the problem area and the active ingredient slowly releases as the polymer breaks down.
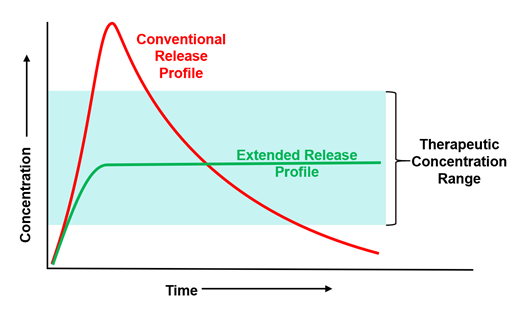
In terms we are all familiar with post-2020, the drug release profile is just like flattening the dose curve.
The technology means fewer injections, less side effects, and a longer pharmaceutical activity window.
Eupraxia‘s injectable is remarkable compared to competitors because it is more than 90% active drug.
IMAGE 1: Flattened dose curve from extended-release polymers
Eupraxia‘s most advanced product is EP-104IAR, an injectable formulation aimed to treat knee osteoarthritis. It is currently in Phase II trials, having passed Phase I safety check. The product uses a well-studied and well-used corticosteroid fluticasone propionate as the active ingredient.
Phase II is expected to be completed in August 2022 and reported a few months later in November 2022, so investors will have to be patient.
EP-104IAR is under investigation for the treatment in dogs too.
IMAGE 2: Technological advantages from Eupraxia’s technology
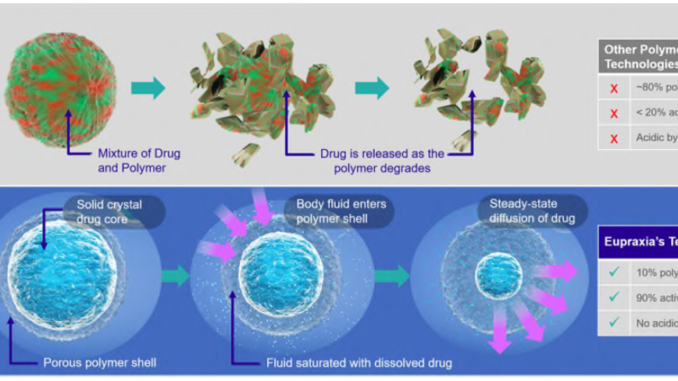
The company has other products in their pipeline to treat post-surgical infections and post-surgical pain. Similar to its flagship injectable, these products encapsulate proven bioactive molecules in a slow-release polymer.


